Tag: Liberty Pro
Anwenderkurse zur Peptid-Synthese im Februar 2024
Die CEM GmbH bietet 2024 Ausbildungs- und Anwenderkurse zur Peptid-Synthese in den eigenen Firmenräumen in Kamp-Lintfort am Niederrhein an.
Im Rahmen dieser Kurse werden die Teilnehmer/innen in den theoretischen Grundlagen und insbesondere in der Methodenentwicklung für die Peptid-Synthese geschult. Bei den praktischen Übungen in kleinen Gruppen können die Teilnehmer/innen anhand der individuellen Problemstellungen Methoden programmieren. Das Ziel dieses Anwenderkurses ist das eigenständige Entwickeln von Synthese-Protokollen bzw. Synthese-Methoden.
Der Teilnehmerkreis richtet sich an Laboranten/innen, Ingenieure/innen, Chemiker/innen, etc. Der erste Seminartag beginnt um 13.30 Uhr, es kann also vorher die Anreise erfolgen. Am 2. Seminartag endet die Veranstaltung um 14.00 Uhr, so dass anschließend die Heimfahrt angetreten werden kann.
In Abhängig der Zusammensetzung der Teilnehmer*innen findet der Kurs in deutscher oder englischer Sprache statt.
Der Kostenbeitrag für dieses 2tägige Seminar beträgt 1.500 EUR zzgl. MWSt. und beinhaltet:
- umfangreiche Seminarunterlagen
- zwei Mittagessen
- ein gemeinsames Abendessen
- eine Hotelübernachtung
- den Transfer zwischen dem Hotel und den Schulungsräumen
- und den Transfer am Seminarende zum ICE-Bahnhof Duisburg oder zum Flughafen Düsseldorf.
Folgende Seminarinhalte werden behandelt:
Übersicht und Grundlagen der Peptid-Synthese-Methodik
- Sicherheitsaspekte
- Applikationsbeispiele
- Maintenance & Troubleshooting, selbst Hand anlegen
- Praktikumsstart, Einweisung an den Geräten, Tipps
Grundlagen zur Methodenentwicklung
- Grundlagen der Reaktionsbedingungen für die optimale Synthese
- Grundlagen der Mikrowellen-Peptid-Synthese (Kurs 1)
- Grundlagen zu den Modulen Platten, Säulen, SPOT (Kurs 2)
- Alternative Lösemittel
- Harze, Eigenschaften und optimaler Einsatz
Praktische Arbeit
Praktische Tipps zur erfolgreichen Synthesestrategie
Termine:
Kurs 1: Anwenderkurs für die mikrowellenaktivierte Peptid-Synthese (Discover SPS, Liberty, Liberty Blue, Liberty Prime) am 21. & 22. Februar 2024
Kurs 2: Anwenderkurs für die parallele Peptid-Synthese (MultiPep) am 28. & 29. Februar 2024
Anmeldung:
Anmeldung Anwenderkurs Peptidsynthese
Hinweis
Auf Wunsch bieten wir nach individueller Absprache auch Anwenderkurse vor Ort beim Kunden an. Zur Terminabsprache und für Detailinfos kontaktieren Sie bitte den Kursleiter Herrn Sengutta unter ulf.sengutta(at)cem.com
Wählen Sie jetzt das PRODUKT DES JAHRES 2023!
Die Laborbranche steckt voller Innovationen, es wird Zeit, diese zu zeigen: Die Leser von LABO können ab sofort die LABO Produkte des Jahres wählen.
https://www.labo.de/leserpreis-2023.htm
Unser Peptidsynthesizer Liberty Blue 2.0 und der schnellste Muffelofen der Welt Phönix Black warten auf Ihre Stimme.
#LibertyBlue #PhönixBlack
#Peptidsynthese #Muffelofen #SchnellerMuffelofen
10. März 2021, ab 10.15 Uhr
Erleben Sie einen ganzen Tag interessante Vorträge zu den Neuigkeiten der Peptid-Synthese. Aufgrund der Corona-Situation können Präsenzvorträge derzeit nicht stattfinden. Deshalb veranstaltet CEM zusammen mit bekannten Forschern/innen dieses kostenfreie Online Seminar.
Academic Guest Speakers:
Professor Anna Maria Papini
University of Florence,
CY Cergy Paris Université

The challenge to design fully automated solid-phase MW-assisted cGMP ready processes of peptide production as active pharmaceutical ingredients
Interdepartmental Research Unit of Peptide & Protein Chemistry and Biology, Departments of Chemistry and NeuroFarBa, University of Florence (Italy)
With the increase of approved peptide-based drugs and patents expiring, the manufacturers’ need in production of peptides is dramatically rising. Companies involved in peptide-based drugs that have to be compliant with good manufacturing practices (GMP), face several challenges, such as high production costs on downstream processes (due to the use of high raw material amounts) and bad scalability. Therefore, the main challenge of production of active peptides as pharmaceutical ingredients is to find solutions to specific problems encountered during scale-up.
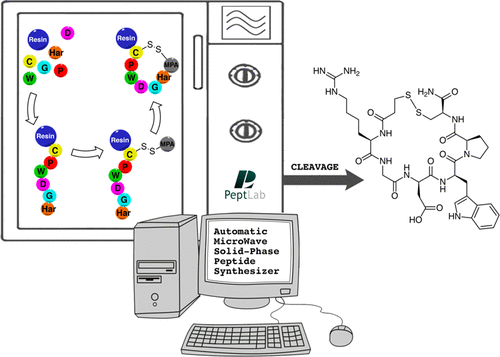
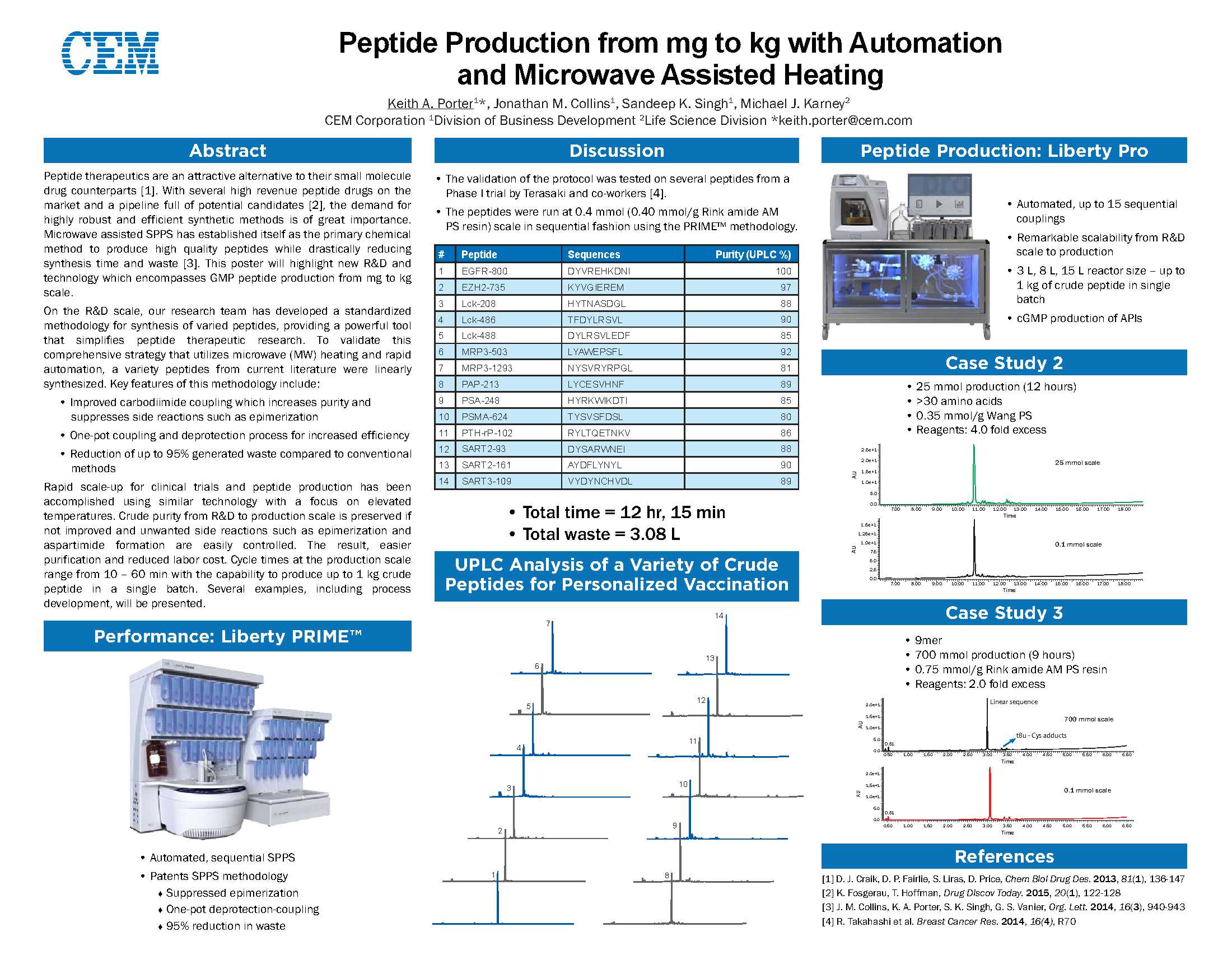
As a proof-of concept we will report the strategies we investigated for the preparation of Eptifibatide, scalable to kilogram-scale, having in common the use of the microwave-assisted solid-phase peptide synthesis (MW-SPPS) procedure, which is now available not only at R&D level but also for the large-scale manufacturing of peptides. Following the very fast microwave-assisted Fmoc/tBu synthesis of the Eptifibatide linear precursor by a DIC/Oxyma Pure coupling protocol at 90 °C, we explored both the solution (off-resin) and the solid-phase (on-resin) disulfide bond formation.
The relevance of the strategy we optimized is essentially based on the selection of the StBu orthogonal protection on cysteine that is easily removed by MPA acting as a novel reducing agent for Cys(StBu) deprotection. Moreover, we demonstrated that all operations can be performed by fully automated process in the instrumentation.
To the best of our knowledge, this is a unique strategy performing all the processes including disulfide bond formation in a single reactor and represents an optimized scalable fully automated solid-phase microwave-assisted cGMP-ready process to prepare Eptifibatide.
Designing Synthetic Peptides to Explore the Dark Matter of Protein Space
Dek Woolfson
Schools of Chemistry and Biochemistry, & Bristol BioDesign Institute
University of Bristol, UK
Protein design—i.e., the construction of entirely new protein sequences that fold into prescribed structures—has come of age: it is now possible to generate a wide variety stable protein folds from scratch using rational and/or computational approaches. A new challenge for the field is to move past protein structures offered up by nature and to target the so-called ‘dark matter of protein space’; that is, protein structures that should be possible in terms of chemistry and physics, but which biology seems to have overlook or not used prolifically. This talk will illustrate what is currently possible in this nascent field using de novo a-helical coiled-coil peptides as building blocks for assembling larger, more-complex and functional protein-like structures.1
Coiled coils are bundles of 2 or more a helices that wrap around each other to form rope-like structures. They are one of the dominant structures that direct natural protein-protein interactions. Our understanding of coiled coils provides a strong basis for building new proteins from first principles. The first part of my talk will survey this understanding,1 our design methods,2,3 and our current “toolkit” of de novo coiled coils.4-5
Next, I will describe how the toolkit can be expanded used to generate some dark-matter protein structures. I’ll focus on the rational and computational design of a-helical barrel proteins, which have 5 or more helices surrounding accessible central channels.6 Finally, I’ll discuss how these synthetic barrel proteins can be put to use to make new nanotube materials,7 rudimentary catalysts,8 membrane-spanning pores,9 and the components of a new types of sensing devices.10
Professor Dek Woolfson
University of Bristol

Professor Fernando Albericio
University of Barcelona,
University of Kwazulu-Natal

Microwave-Assisted Solid-Phase Peptide Synthesis, A Unique Tool for Peptide Research
Fernando Albericio,1,2 Beatriz G. de la Torre1
1University of KwaZulu-Natal, Durban 4001, South Africa; 2University of Barcelona, 08028-Barcelona, Spain
During the last five years (2016-2020), 18 drugs containing peptides have been approved by the US FDA. In addition, several hundred are in clinical phases or advanced preclinical studies. The development of new synthetic strategies has facilitated this explosion in the world of peptides. In this regard, the development of new resins, coupling reagents, protecting groups, and, more importantly, reliable and robust peptide synthesizers have been vital for the consolidation of the peptide drug market.
In the field of automatic synthesizers, the last breakthrough occurred when at the beginning of this century, CEM launched the new concept of Microwave-Assisted SPPS. Our group and others have demonstrated that both couplings and Fmoc-removal are better performed in the microwave mode compared with classical synthesis or even with conventional heating. More important, this better performance in the two key reactions is not accompanied by any significant side-reaction.
This presentation will discuss our last results on the use of Microwave synthesis for the preparation of intriguing peptides and/or for developing new synthetic strategies: (i) the preparation of staple peptides via late-stage C(sp2)-H Pd activation using the microwave; (ii) synthesis of N-alkyl amino acid containing peptides; (iii) development of new and more efficient coupling reagents; and (iv) microwave-based Green Solid-Phase Peptide Synthesis (GSPPS).
In the GSPPS, the CEM Liberty Blue Microwave technology by itself could be considered green in terms of solvent consumption and time-saving and due to the excellent quality of the crude peptides, which enormously facilitates the purification with the consequent increase of yield and reduction of chromatography solvents.
Short antimicrobial peptides – discovery and optimization
Kai Hilpert, St George’s University
The global health threat surrounding bacterial resistance has resulted in antibiotic researchers shifting their focus away from ‘traditional’ antibiotics and concentrating on other antimicrobial agents, including antimicrobial peptides.
These peptides exhibit broad-spectrum activity against bacteria, including multi-drug resistant strains, viruses, fungi, and protozoa, and constitute a major element of the innate immune system of many multicellular organisms.
We use the Spot-synthesis technique to study and optimize naturally occurring as well as artificial antimicrobial peptides. Understanding the features that make them antibacterial and hemolytic allows for targeted drug development.
The authors investigated several strategies, based on the use of microwave-assisted solid-phase peptide synthesis (MW-SPPS) and scalable to kilogram-scale manufacturing, for the preparation of Eptifibatide, a disulfide-bridged cyclo-heptapeptide drug approved as an antithrombotic agent. Following the very fast microwave-assisted Fmoc/tBu synthesis of the linear precursor, we explored both the solution (off-resin) and the solid-phase (on-resin) disulfide formation. In order to optimize the oxidation in solution, we focused our attention on the mild disulfide formation procedure based on the use of air, observing some drawbacks, such as the formation of unwanted oxidation byproducts, such as dimers, or the use of large volumes of an environmentally unfriendly solvent (CH3CN). In order to overcome these difficulties, we studied four different on-resin strategies, with the final aim to develop a fully automated, single reactor procedure, exploring different strategies to protect the thiol side-chain functional group on the C-terminal Cys residue and to form the Eptifibatide ring. The main difference among these strategies is represented by the final cyclization mode that was obtained either by direct formation of an S–S disulfide bridge or by head to MPA on cysteine side-chain amide bond formation. In conclusion, the optimization of the latter strategy enabled us to devise an optimized scalable fully automated solid-phase microwave-assisted cGMP-ready process to prepare Eptifibatide.
Im kostenfreien Web-Seminar erläutern die Referenten Frau Dr. Monika Swiontek und Herr Dr. Christian Behn die unterschiedlichen Techniken zur schnellen und flexiblen Peptid-Synthese.
Die Synthese unter Mikrowellenaktivierung ermöglicht in wenigen Stunden die Darstellung reiner Peptide statt wie üblich in vielen Tagen. Synthesemaßstäbe von Milligramm Mengen bis zur Produktion im kg-Bereich werden vorgestellt. Während einer live Vorführung im Labor erleben Sie einen Kopplungszyklus und lernen dabei die einfache intuitive Software kennen. In Ergänzung dazu werden flexible Formate der multiplen parallelen Synthese vorgestellt, wie z. B. SPOT-Synthese, Festphasensynthese in 96er Filterplatten- und Filtersäulen zur Synthese von Peptid- und PNA-Bibliotheken. Zusätzlich wird ein Aspekt der beiden Referenten auf die Abspaltung der fertig synthetisierten Peptide gelegt und es werden zwei moderne Cleavage-Systeme vorgestellt.
Mittwoch, den 9. Dezember 2020, 10.00 – 11.30 Uhr und 14.00 – 15.30 Uhr
Mikrowellen-Peptidsynthese der 2. und 3. Generation Mehr erfahren