Our Blog
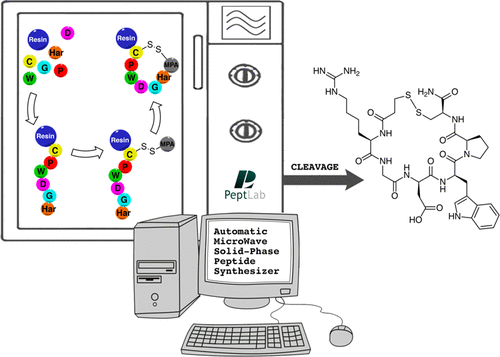
The authors investigated several strategies, based on the use of microwave-assisted solid-phase peptide synthesis (MW-SPPS) and scalable to kilogram-scale manufacturing, for the preparation of Eptifibatide, a disulfide-bridged cyclo-heptapeptide drug approved as an antithrombotic agent. Following the very fast microwave-assisted Fmoc/tBu synthesis of the linear precursor, we explored both the solution (off-resin) and the solid-phase (on-resin) disulfide formation. In order to optimize the oxidation in solution, we focused our attention on the mild disulfide formation procedure based on the use of air, observing some drawbacks, such as the formation of unwanted oxidation byproducts, such as dimers, or the use of large volumes of an environmentally unfriendly solvent (CH3CN). In order to overcome these difficulties, we studied four different on-resin strategies, with the final aim to develop a fully automated, single reactor procedure, exploring different strategies to protect the thiol side-chain functional group on the C-terminal Cys residue and to form the Eptifibatide ring. The main difference among these strategies is represented by the final cyclization mode that was obtained either by direct formation of an S–S disulfide bridge or by head to MPA on cysteine side-chain amide bond formation. In conclusion, the optimization of the latter strategy enabled us to devise an optimized scalable fully automated solid-phase microwave-assisted cGMP-ready process to prepare Eptifibatide.