Our Blog
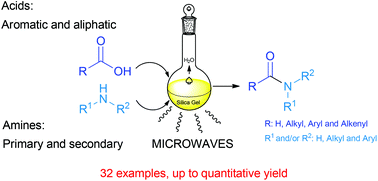
A highly improved and green methodology for the direct amidation of carboxylic acids with amines using silica gel as a solid support and catalyst is described. The scope of this method is exemplified by the use of several aliphatic, aromatic, unsaturated and fatty acids. The reaction is also applied to different primary and secondary amines. Typically, the amines should be aliphatic, but aromatic amines can be used as well, though with lower yields. Several experiments to illustrate the selectivity of this methodology were also carried out with several more functionalized acids and amines. This approach is a substantial improvement over other previously described methods in amide synthesis.
Amine (1.5 mmol) and carboxylic acid (1.5 mmol) were dissolved in ethyl acetate (15 mL), then silica gel 60 230-400 mesh (1.0 g) was added. The solvent was removed under reduced pressure, the reactant mixture was transferred to a microwaves tube, and it was set to react in a CEM microwave reactor in cycles of 20 minutes at a power of 200 W maintaining constant temperature at 130 °C, and a hold time of 2 minutes. The reactant mixture was allowed to cool to room temperature, sonicated for 20 minutes with 30 mL of ethyl acetate, filtered, and the silica was washed with another 30 mL of ethyl acetate. The organic phase was washed with a saturated solution of NaHCO3 and HCl (10%), dried over MgSO4, filtered and the solvent was withdrawn under reduced pressure to obtain the pure product. In some cases flash chromatography was and it is indicated in the characterization data.